At present, the global incidence of chronic kidney disease exceeds 10%, of which 90% is caused by glomerular disease. Podocyte injury is now appreciated to be at the crux of many forms of proteinuric kidney diseases and podocyte injury in glomerular disease is considered as the pathophysiological basis for proteinuria, glomerulosclerosis and progressive deterioration of renal function. In view of this, the identification of novel pathophysiological pathways and molecules in podocytes is essential for the prevention of glomerular disease progression and the discovery of new avenues for treatment. Although mature podocytes lack tight junctions (TJs), immunofluorescence and immunoelectron microscopy analysis showed that CLDN5 is the major CLDN expressed on the plasma membranes of mature podocytes under normal conditions. So far, little is known about the functional role of CLDN5 in glomerular physiology and disease development.
On March 24, 2022, the Kidney Research Team of our school published a research article titled Loss of CLDN5 in podocytes deregulates WIF1 to activate WNT signaling and contributes to kidney disease in Nature Communications. The present study reveals a previously undescribed function and an important regulatory role for the tight junction protein CLDN5 deregulates WIF1 to activate WNT signaling and contributes to kidney disease.

Using a new mouse model in which the Cldn5 is specifically deleted in podocytes, the authors identify CLDN5 as a crucial regulator of podocyte function and show that podocytes specific Cldn5 deficiency exacerbates podocyte injury and proteinuria in diabetic nephropathy mouse model. Moreover, podocyte CLDN5 deletion also aggravated ureteral obstruction-induced renal fibrosis in mice, which suggested that podocyte CLDN5 plays a protective role in kidney disease.
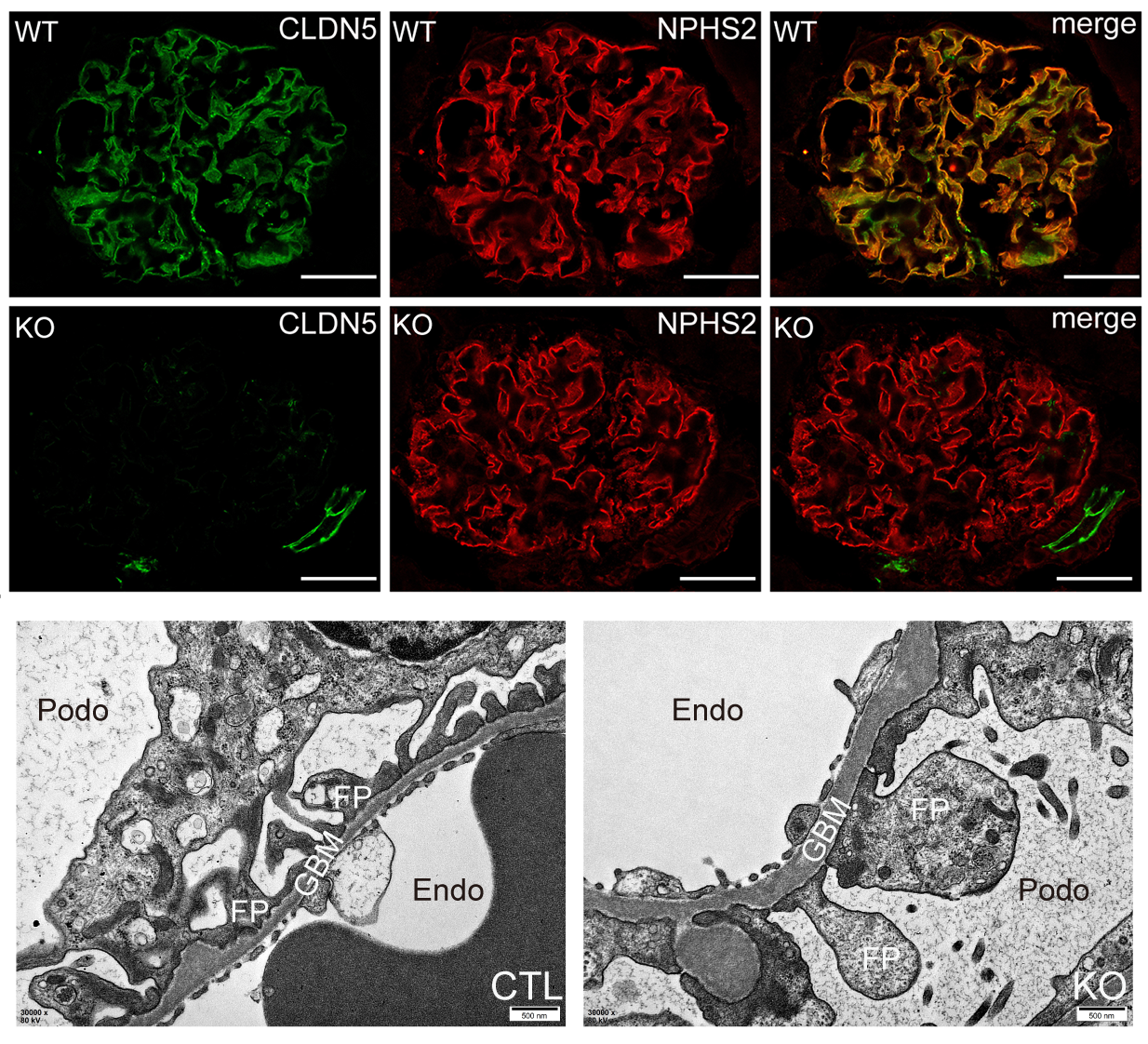
Analysis of podocyte-specific Cldn5 knockout mice
The authors also identify for the first time the glomerular expression of WIF1, which is largely restricted to podocytes, decreased in diabetic nephropathy. Knockout Wif1 in podocytes result in the development of proteinuria and the typical ultrastructure change occurring in Cldn5 knockout mice, while targeted delivery of WIF1 to podocytes prevents the development of glomerular nephropathy in the Cldn5 knockout diabetic mice. These findings describe a novel role of CLDN5 in its capacity to avert WNT-signaling hyperactivation in podocytes via transcriptional regulation of WIF1 expression.
Through co-immunoprecipitation, subcellular fraction extraction, immunofluorescence co-localization and luciferase reporter analysis, the authors confirmed that CLDN5 forms a complex with ZO1 and ZONAB in normal podocytes under physiological conditions, and this complex is required to sustain ZONAB’s subcellular localization. CLDN5 absence reduces ZO1 expression and induces the nuclear translocation of transcription factor ZONAB, followed by transcriptional downregulation of WIF1, which leads to activation of WNT signaling pathway.
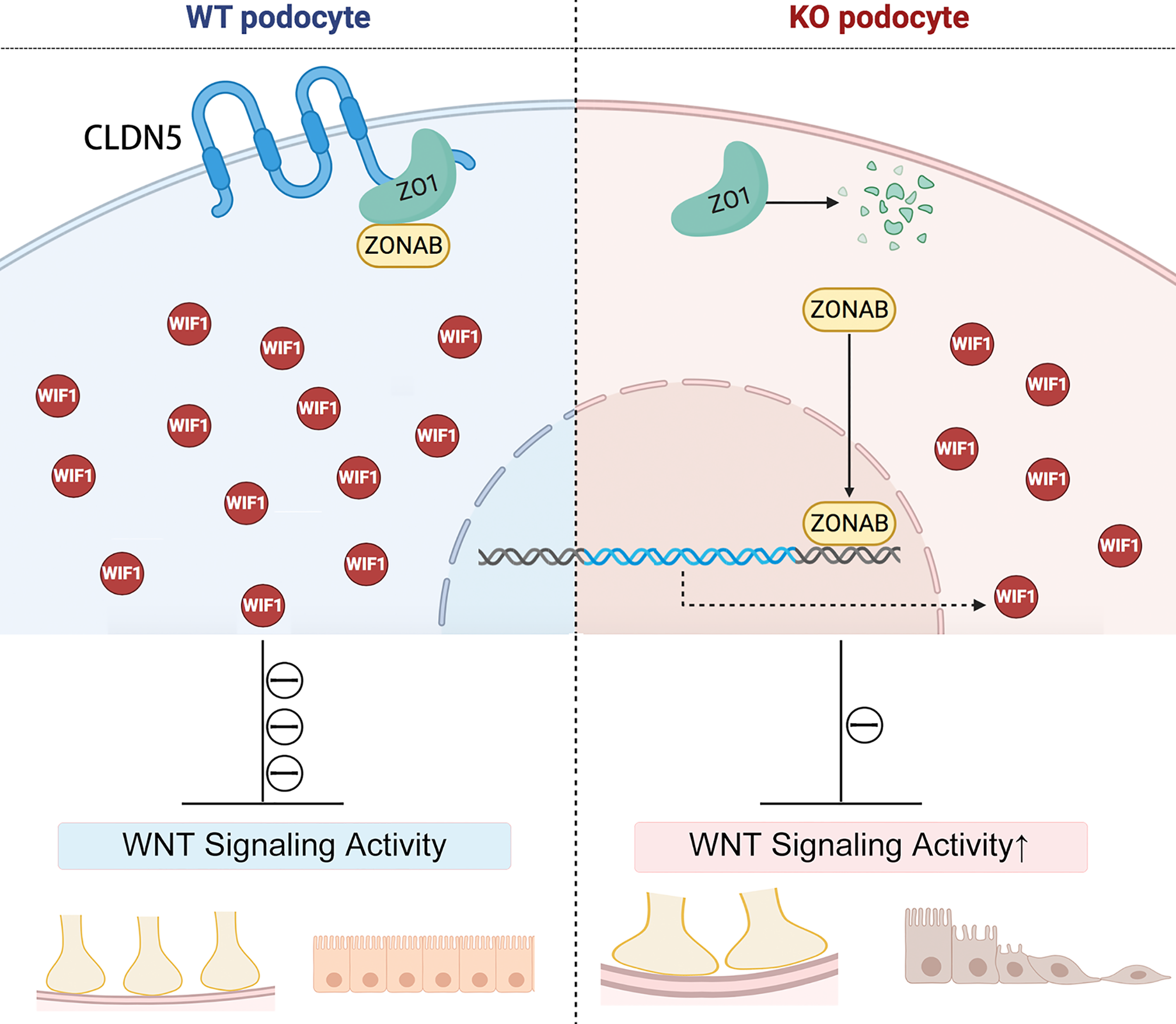
Podocyte CLDN5 regulates Wif1 expression and involves in the development of kidney disease
This study provides new insights regarding the role of CLDNs and tight junction in kidney under physiological and pathological conditions that will be critical to understand a broad range of epithelial biology. These exciting observations should also stimulate the investigation of CLDN5 and WIF1 as therapeutic targets to prevent or treat chronic kidney disease affecting millions of people worldwide.
Associate Professor Hui Sun from the Department of Physiology and postgraduate student Hui Li and Jie Yan are the first authors of this paper. Professor Yongfeng Gong is the corresponding author of this paper. This research was supported by grants from the National Natural Science Foundation of China and Start-up of Binzhou Medical University.
Link to the research article:
https://www.nature.com/articles/s41467-022-29277-6
Source: Department of Science and Technology





